Abstract
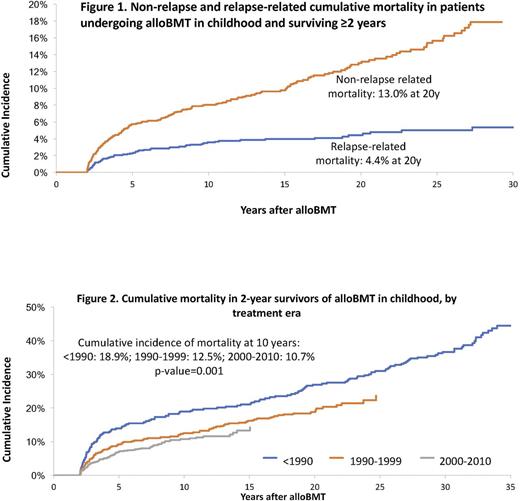
Background: AlloBMT is a curative option for children with malignant and non-malignant diseases. Nonetheless, the high intensity of therapeutic exposures at a young age increases the risk of organ compromise and may lead to premature death. However, there remains a gap in knowledge regarding cause-specific late mortality experienced by children undergoing alloBMT. Furthermore, it is not clear whether mortality rates have changed over the past 4 decades. We address these gaps by examining premature death (all-cause, relapse-related mortality [RRM] and non-relapse-related mortality [NRM]) in patients undergoing alloBMT between 1974 and 2010 at City of Hope, University of Minnesota or University of Alabama at Birmingham. Methods: To be included in this analysis, the patients had to have received alloBMT before age 22, and survived for ≥2y after transplantation. Vital status information was ascertained as of Dec 31, 2016, using medical records, the National Death Index (NDI), and Accurint databases. Information on cause of death was obtained from the NDI Plus program and medical records. Using Kaplan-Meier techniques, we describe overall survival conditional on surviving 2+ and 20+y from alloBMT. We describe cumulative incidence of RRM and NRM using competing risk methods. We also describe all-cause late mortality for patients transplanted over 3 time periods: <1990; 1990-1999; 2000-2010. Standardized mortality ratio (SMR) was used to compare the mortality experienced by this cohort to the age- sex-, and calendar-specific mortality of the US general population. Proportional subdistribution hazards model (Fine-Gray) for competing risks was used for identifying predictors of late mortality, including demographics, primary disease, conditioning agents, disease status at alloBMT and GvHD prophylaxis. Results: In this cohort of 1,411 recipients of alloBMT performed in childhood and surviving ≥2y, the most common primary diagnoses were acute lymphoblastic leukemia (ALL: 25%), acute myeloid leukemia/myelodysplastic syndrome (AML/MDS: 24%), inborn errors of metabolism (IEM: 14%) and severe aplastic anemia (SAA: 11%). The distribution of the cohort over the 3 time periods was: <1990 (23%); 1990-1999 (29%); 2000-2010 (48%). After a median follow-up of 14.9y, we observed 295 deaths in this 2y survivor cohort, yielding an overall survival rate of 79.6% at 20y from alloBMT. The leading causes of premature death were infection (32.0%), primary disease (24.6%) and subsequent malignant neoplasms (18.4%). Overall, the cohort was at a 15-fold increased risk for premature death (SMR 15.3, 95% CI 13.6-17.2), when compared with the general population. Conditional on surviving 20y, the 5y overall survival was 96.0% for the entire cohort, and 98.4% for SAA, 96.0% for AML/MDS, 90.3% for ALL and 85.8% for IEM. Relative mortality declined with time from alloBMT, but remained significantly elevated at 25y after transplantation (SMR 1.9, 95% CI 1.1-2.9). The cumulative incidence of NRM exceeded that due to RRM (fig 1); the 20y cumulative incidence of NRM and RRM were 13.0% and 4.4%, respectively. The all-cause 10y cumulative mortality rate declined over the 3 eras (<1990: 18.9%, 1990-1999: 12.5%; >2000: 10.7%, p=0.001) (Fig 2). Adjusting for demographic and clinical variables, as well as conditioning, the hazard ratio (HR) of all-cause death decreased with more recent treatment eras (<1990: HR=1.0; 1990-1999: HR=0.7, 95%CI, 0.5-0.9, p=0.01; 2000-2010: HR=0.6, 95%CI, 0.4-0.8, p=0.004). Conclusions: This study demonstrates that compared with the general population, 2y survivors of alloBMT performed in childhood remain at an elevated risk of premature death, even after 25y from alloBMT. While the risk of relapse-related mortality plateaus with time, non-relapse-related mortality continues to increase and remains the major cause of late death in this population. However, we make an encouraging observation i.e., there has been a significant decline in all-cause mortality experienced by children undergoing alloBMT over the past 4 decades.
Arora: Takeda Oncology: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal